Recall on EpiPen batches after possible default
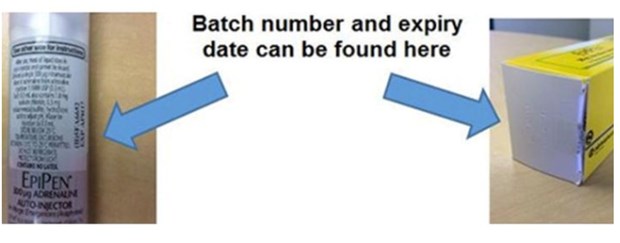
Check your batch number and expiry date.
By Practical Parenting
March 21 2017
Four batches of EpiPens are being recalled.
The Therapeutic Goods Administration is urging people to check the batch number on their EpiPens distributed, worldwide by Mylan-owned Alphapharm, after concerns they contain a defect and won’t inject properly.
"The failure of the auto-injector to activate may result in patients not receiving the required dose of adrenaline, resulting in the worsening of symptoms of anaphylaxis or anaphylactic reactions, which could be life-threatening," the company said.
EpiPens are used to treat people suffering severe allergic reactions. There have been two reports of EpiPens not injecting properly.
People with the EpiPen 300 microgram adrenaline injection syringe auto-injectors have been asked to check the batch number and expiry date.
Those from batches 5FA665, 5FA6651, 5FA6652 and 5FA6653, all expiring in April 2017, should return them to any pharmacy to swap for one from a different batch for free.
No other batches of EpiPen are thought to be affected.
Alphapharm said: "At this time, EpiPen Jr 150 microgram adrenaline injection syringe auto-injectors and all other batches of EpiPen 300 microgram adrenaline injection syringe auto-injectors are unaffected and are not subject to this recall."
It is also advised that people with recalled batch numbers keep devices until they can be replaced, "being mindful that you may need to apply more force than normal to activate it".






